
In numerous tests comparing OSDrC® to the performance of conventional tableting machines and reproducibility of the tablets, OSDrC® technology has proven itself better than existing tablet technology.OSDrC® technology and equipment are protected by patents and the overall patent estate is continuously being expanded to fully protect the technology.
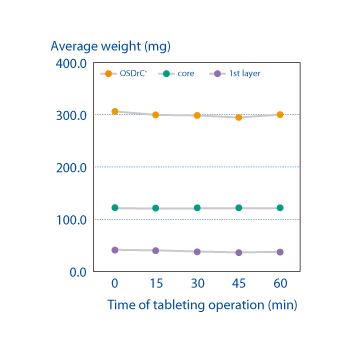
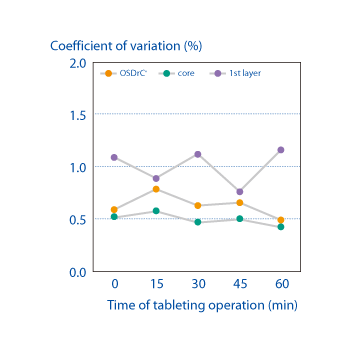
Measurements of the average mass of the coating, core, and tablet, and temporal measurement of the coefficient of variance, have revealed that OSDrC® tableting machines deliver superior results to conventional tableting machines in term of mass variability.
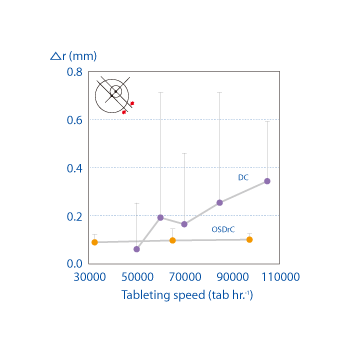
Whereas misalignment occurs in conventional tableting machines as the speed of output increases, tests comparing OSDrC® rotary tableting machines and conventional tableting machines show extremely low core misalignment (0.1mm) with the OSDrC® rotary tableting machine.
Ozeki, Journal of Japan Society of Pharmaceutical Machinery and Engineering, 2005, 14(4), 12-21
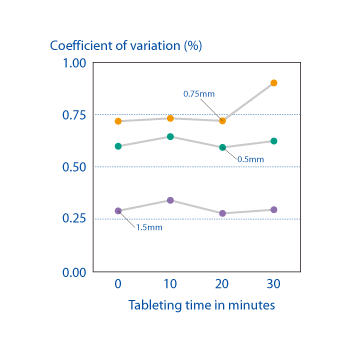
Measurements of the coefficient of variance in cored tablets produced on an OSDrC® rotary tableting machine, where the tablets have a coating thickness of 0.5 mm or 0.75 mm, reveal little mass variability and demonstrate that OSDrC® tableting machines can produce individual coatings that are comparable to film coating tablets.

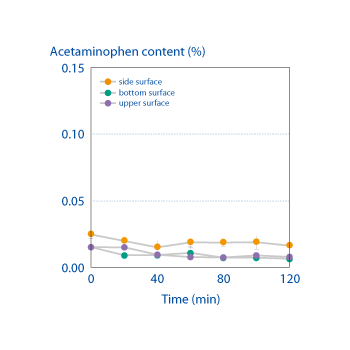
The top, side, and bottom of the coating on tablets produced by an OSDrC® rotary tableting machine were each measured for cross-contamination with the core substance. These measurements demonstrated that the amount of API (acetaminophen) that migrated from the core to the coating was extremely low (less than 0.03%). OSDrC® technology is clearly effective at controlling the interaction of the API and the coating excipient.
Ozeki, Journal of Japan Society of Pharmaceutical Machinery and Engineering, 2005, 14(4), 12-21
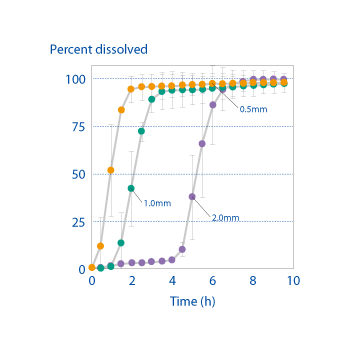
Measurements of the release rate of the core API (acetaminophen) in dividable tablets produced on an OSDrC® rotary tableting machine, where the tablets have a coating thickness of 0.5 mm, 1.0 mm, or 2.0 mm, verify that the release of the API can be controlled by the thickness of the coating.
Ozeki et al., Journal of Controlled Release, 2004, 95(1), 51-60
In contrast to existing tableting technology, where dividing an enteric coated tablet would lead to performance failure, the OSDrC® rotary tableting technology is capable of producing enteric-coated dividable core tablets that maintain performance after dividing the tablet. Measurements of the API (acetaminophen) release rate from the core in dividable tablets produced on an OSDrC® rotary tableting machine verified that the release characteristics were the same before dividing and after dividing.

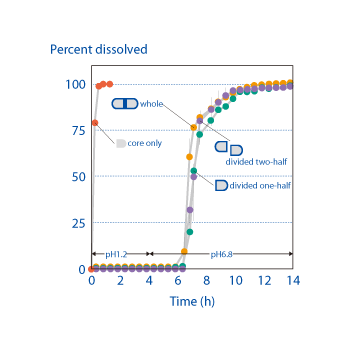
Ozeki et al., Pharmaceutical Research, 2004, 21(7), 1177-1183
When the core of an OSDrC® tablet was prepared with a poorly compressible API powder (acetaminophen crystals) and the friability measured, it was verified that OSDrC® tablets had better friability than those manufactured by direct compression of a physical mixture (PM).

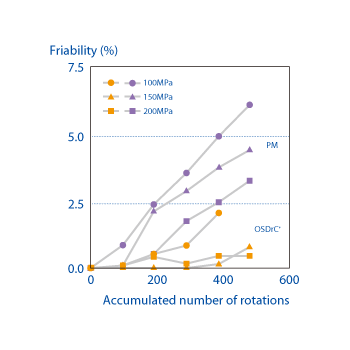
Ozeki et al., International Journal of Pharmaceutics, 2003, 259(1-2), 69-77
Because OSDrC® technology can use even substances with poor compressibility as the core, it can replace encapsulated drugs with tablets. Measurements of the release characteristics of a divided tablet containing pellets, where the tablet was produced on an OSDrC® rotary tableting machine, revealed that the divided tablet demonstrated the same release characteristics as the original encapsulated drug.
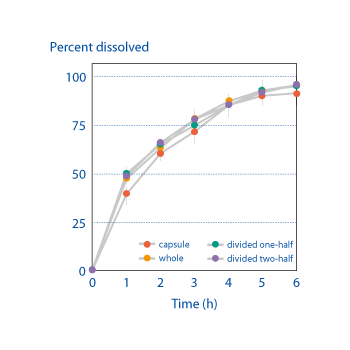
SKK company data, 2004